Law Of Multiple Proportions Worksheet Doc
Two unknown compounds are tested. Get thousands of teacher-crafted activities that sync up with the school year.

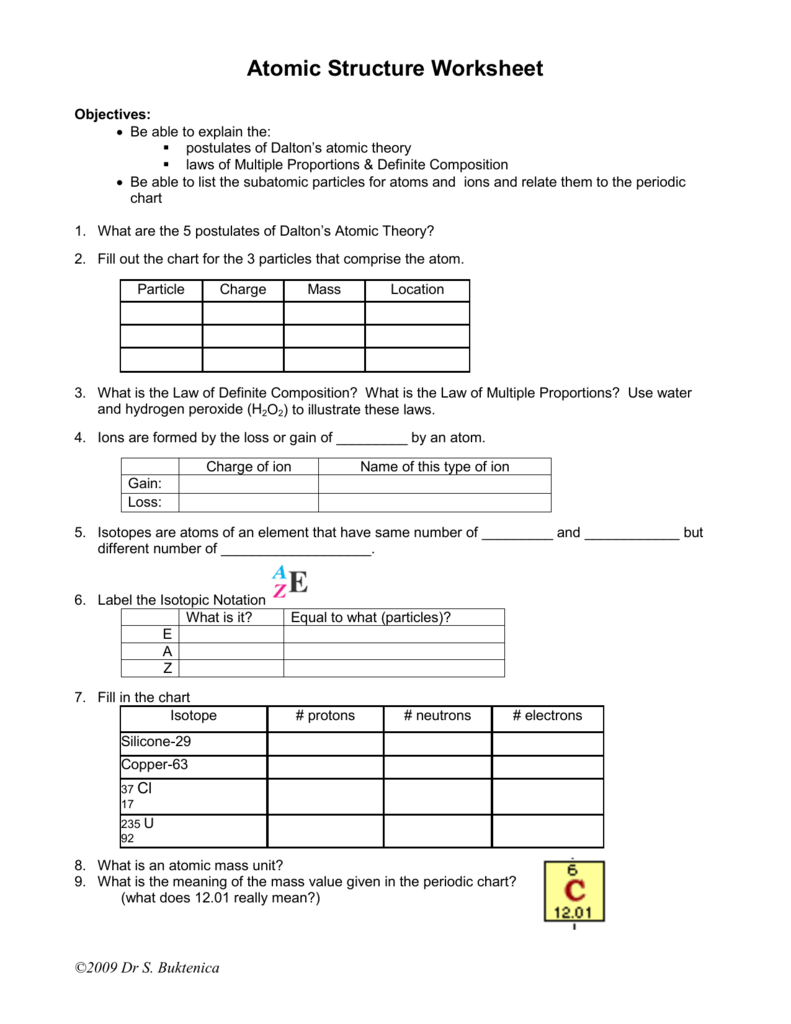
Law Of Definite Proportions Docx Worksheet Law Of Definite Proportions Directions Show All Work 1 A 78 0 G Sample Of An Unknown Compound Contains 12 4 Course Hero
Ad Learn 3000 maths skills online.

Law of multiple proportions worksheet doc. U nderstand a tomic weight and the significance of its unit amu. 1 gram of the first element can always be reduced to small whole numbers. Rutherfords gold foil experiment.
Pure water always contains 11 Hydrogen and 89 Oxygen. Worksheet 22. Law of definite proportions.
Get thousands of teacher-crafted activities that sync up with the school year. Compound I contains 150 g of hydrogen and 1200 g of oxygen. Other scientists followed up on the law of conservation of mass by stating the.
Atoms Molecules and Ions 1. An atomic theory based on these laws was developed by __7__. Draw and describe the atom model of each of the following scientists.
Writing nuclear symbols of atoms and ions. Microsoft Word - Worksheet-Law of Definite Proportionsdoc Author. Law of Multiple Proportion - Free download as PDF File pdf Text File txt or read online for free.
Law of Conservation of Mass Matter is neither created nor destroyed in a chemical reaction molecules change to create new substances. Compounds are pure substances that contain two or more elements. John Dalton was a strong proponent of the Law of Multiple Proportions which is the 3rd postulate of Dalton.
Briefly define the following. Law of multiple proportions. The law of __6__ proposed soon after states that the masses of one element that combine with a fixed mass of another element in different compounds are in simple whole-number ratios.
THE LAW OF DEFINITE PROPORTIONS. Composed of two or more transition elements b. Ad The most comprehensive library of free printable worksheets digital games for kids.
An Example of the Law of Multiple Proportions Problem 2-14 Phosphorus forms two compounds with chlorine. C the law of conservation of mass. Law of Multiple Proportions.
Compound I contains 150 g of hydrogen and 1200 g of oxygen. Yes it follows the law of multiple proportions. Law of Definite Proportions.
Thomsons cathode ray tube experiments. Explain how the previous examples help to illustrate the Law of Definite Proportions. A the law of multiple proportions.
Compound II contains 20 g of hydrogen and 320 g of oxygen. B the law of constant composition. 1 A molecule of water contains hydrogen and oxygen in a 18 ratio by mass.
Parents trust IXL to help their kids reach their academic potential. Parents trust IXL to help their kids reach their academic potential. These hypotheses explained three fundamental laws.
Compound II contains 20 g of hydrogen and 320 g of oxygen. The Law of Definite Proportions The Law of Multiple Proportions and The Law of the Conservation of Mass. H 2O 2 Example.
When two elements combine to form two or more new compounds the mass of each of the elements that combine in a way that their ratios are whole numbers. A 34g 168 g 202 g c 34g 336g 370 g. Which of the following.
Law of multiple proportions. Law of multiple proportions When two elements form a series of compounds the ratios of the masses of the second element that combine with a fixed mass ex. Chapter 2 Worksheet page 1 Chapter 2 Worksheet.
No it does not follow the law of multiple proportions. Ad Learn 3000 maths skills online. Law of constantdefinite composition.
Ad The most comprehensive library of free printable worksheets digital games for kids. Law of Multiple Proportions. Calculating the number of protons electrons and neutrons in atoms and ions.
Two compounds of hydrogen and oxygen are tested. This is a statement of _____. D the law of conservation of.
Composed of positive and negative ions c. Exceptions to the law of definite proportions ____ 22. Combined together in fixed or definite proportions.
Law of conservation of mass. Water is an example of this law. Every pure sample of a particular compound always contains the same proportion by mass of the elements in the compound.
In naming a binary molecular compound the number of atoms of each element present in the molecule is indicated by ____. What was the significance of each of the following experiments. Show that these results are consistent with Daltons law of multiple proportions.
Determine the ratio of the mass of oxygen to the mass of hydrogen in each of the. Law of Definite Composition. Are the compounds the same.
In the first compound 1000 g of phosphorus is combined with 3433 g of chlorine and in the second 2500 g phosphorus is combined with 14306 g chlorine. Composed of two or more nonmetallic elements d. Explain each of the laws in your own words.
Yes it follows the law of multiple proportions.
Https People Wou Edu Postonp Ch221 Pdf Law Of Multiple Proportions Pdf
Law Of Multiple Proportions Worksheet
Law Of Multiple Proportions Worksheet

Chemistry For Changing Times Atoms 2 1 Multiple Choice Questions
Laws Of Definite Multiple Proportions
Law Of Multiple Proportions Worksheet

1 1 2 Law Of Constant Composition Or Definite Proportion Proust Pdf Free Download

Answer Key For Hw Problems Chapter 2 Doc Home Work Key Chapter 2 2 2 State The Laws Of Definite Proportions And Multiple Proportions Illustrate Each Course Hero

Law Of Definite Proportions Worksheets Teaching Resources Tpt

Law Of Definite Proportions Docx Worksheet Law Of Definite Proportions Directions Show All Work 1 A 78 0 G Sample Of An Unknown Compound Contains 12 4 Course Hero

Quiz Worksheet Law Of Definite Proportions Study Com

Worksheet 8 Graham 09 Due Pdf Free Download

Law Of Multiple Proportions Law Of Multiple Proportions Proportion Chemistry

Fundamental Laws Of Chemistry Pdf Free Download
Https Misterchemistry Com Wp Content Uploads 2018 10 Law Of Definite Proportion 2 Pdf

1 1 2 Law Of Constant Composition Or Definite Proportion Proust Pdf Free Download
Law Of Multiple Proportion Sets Of Chemical Elements Materials